Increased adhesion of blast cells in acute myeloid leukaemia
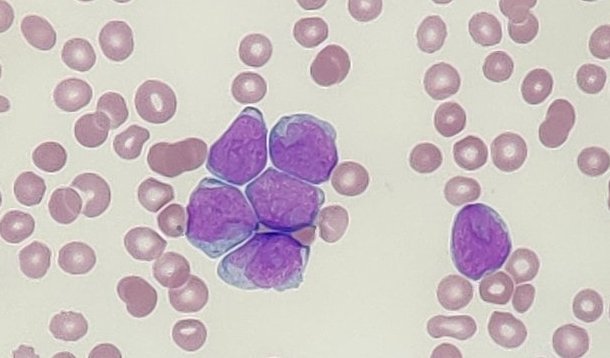
Acute myeloid leukaemia (AML) is a malignancy marked by the proliferation of immature myeloid cells, known as blast cells, within the bone marrow. A notable feature of AML is the increased adhesiveness of these blast cells, leading them to adhere more firmly to each other and to the bone marrow microenvironment. This enhanced adhesion plays a pivotal role in disease progression and therapeutic resistance.
In normal haematopoiesis, cell adhesion molecules (CAMs) regulate essential processes such as cell migration, homing, and maintenance of quiescence within the bone marrow niche. AML blast cells express many of the same CAMs as their healthy counterparts; however, their expression patterns are often dysregulated, reflecting the heterogeneity of the disease. This aberrant expression alters the interactions between leukaemic cells and the bone marrow microenvironment, contributing to increased cell-to-cell adhesion and adhesion to the extracellular matrix.
Several molecular mechanisms underlie the heightened adhesiveness of AML blast cells. One key factor is the overexpression of selectins and their ligands. Selectins are a family of CAMs that mediate the initial tethering and rolling of cells on the vascular endothelium. In AML, blast cells often exhibit increased expression of selectin ligands, facilitating stronger adhesion to the endothelium and promoting leukostasis – a condition characterised by the accumulation of leukaemic cells in small blood vessels, leading to impaired blood flow and tissue damage.
Integrins, another group of CAMs, also play a significant role in AML cell adhesion. These transmembrane receptors mediate firm adhesion to the extracellular matrix and intercellular interactions. AML blasts often display altered integrin expression, enhancing their binding affinity and contributing to increased retention within the bone marrow niche. This retention provides a protective environment, shielding leukaemic cells from chemotherapeutic agents and contributing to minimal residual disease and relapse.
Cytokines and chemokines within the bone marrow microenvironment further modulate the adhesive properties of AML blast cells. Leukaemic cells can secrete tumor necrosis factor-alpha (TNF-α) and interleukins, which upregulate the expression of CAMs on both leukaemic and endothelial cells. This cytokine-driven activation enhances the adhesive interactions, promoting the clustering of blast cells and their adherence to the bone marrow stroma.
The increased adhesiveness of AML blast cells has significant clinical implications. Enhanced cell-to-cell and cell-to-matrix adhesion facilitate the survival and proliferation of leukaemic cells within the bone marrow niche, contributing to disease progression and resistance to therapy. Moreover, the strong adhesion to the endothelium can lead to complications such as leukostasis, increasing the risk of organ damage and failure.
Understanding the mechanisms behind the increased adhesion of AML blast cells offers potential therapeutic avenues. Targeting CAMs or their signaling pathways may disrupt the protective interactions between leukemic cells and the BM microenvironment, enhancing the efficacy of chemotherapeutic agents and reducing the likelihood of relapse. Additionally, interventions aimed at modulating cytokine activity could diminish the adhesive properties of blast cells, potentially alleviating complications associated with leukostasis.
Privacy Policy | Refund & Return Policy | Only Cells LTD © 2025
